Reagents to Photocontrol Microtubules
The cellular scaffolding protein tubulin is the monomeric unit that forms the complex, highly dynamic network of cellular microtubules - gigantic, noncovalent polymeric assemblies that act as roads for cellular trafficking, parking lots for resting proteins, and as girders for applying or sensing forces within (and outside of) cells. Microtubules are continuously being built up, torn down, modified with chemical flags (PTMs), and coordinated by a host of proteins into rapidly changing shapes. These remodelling dynamics are crucial to the correct functioning of many anisotropic cellular processes, most famously, to the separation of genetic material and daughter cells during mitosis/meieosis. Many of the most powerful anticancer drugs (taxanes, vinca alkaloids, discodermolides) work by altering these microtubule dynamics so that cells can no longer divide correctly and instead go into apoptosis.
Our research has pioneered photoisomerisable microtubule inhibitors, that can be precisely controlled through reversible optical patterning. These photopharmaceutical antimitotics are allowing researchers to use light to reversibly modulate microtubule-dependent processes in eukaryotes, in 2D and 3D cell culture, as well as in vivo across model organisms from worm and fly to fish, frog, and mouse: with applications in fields from cargo transport to cell migration, cell division, and embryonic development. These results were made possible by merging the right molecular photoswitches, several of which we have pioneered, with the appropriate drug cores: and taking the resulting photopharmaceuticals through cellular to early in vivo biological testing. We are now stretching the chemical space of antimitotics and photoswitches to reach new generations of photoswitchably bioactive drugs with improved potency, in vivo compatibility, and other layers of functionality including subcellular localisation and tissue-retention.
A biologist's guide to MT photocontrol: Thorn-Seshold & Meiring 2022 in Methods in Molecular Biology (Springer).
A chemist's guide to photoswitchable antimitotics: Thorn-Seshold 2022 in Molecular Photoswitches (Wiley).
Swiss National Radio's popular science view on photopharmacology informing drug design (@18:15; OTS @ 25:00).
For our full list of papers on MT reagent development and applications, see the Publications page.
Primary research highlights include:
 |
 |
 |
 |
| Optimised SBTubs: in vivo MT photocontrol (JACS 2022) |
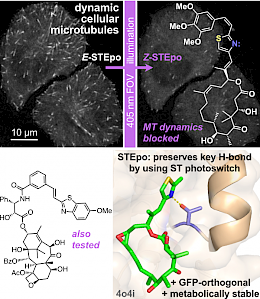
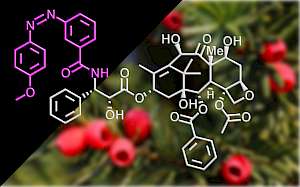
STEpo: epothilone MT photoswitch stabilisers (Angewandte VIP 2021) |
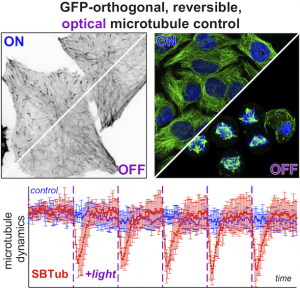
SBTub: GFP-orthogonal MT photoswitches (Cell Chem Biol 2021) |
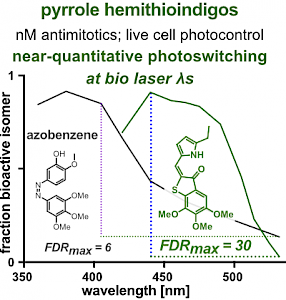
PHTub: Laser-adapted MT photocontrol reagents (Angewandte 2021b) |
|
|
|
||
 |
 |
 |
 |
| AzTax: taxol-based MT photoswitch stabilisers (Nat Commun 2020) |
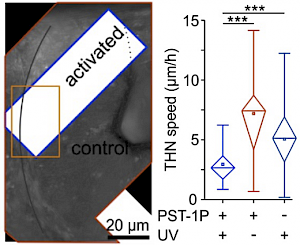
MT photocontrol in cell migration (J Cell Biol 2020b) |
HITub: high-potency HTI-based MT inhibitors (Beilstein JOC 2020) |
MT photocontrol in neurogenesis (J Cell Biol 2020a) |
|
|
|
||
 |
 |
 |
 |
| HOTub: HTI-based photoswitches for MTs (ChemBioChem 2019) |
MT photocontrol in fly tissue morphogenesis (Nat Cell Biol 2018) |
MT photocontrol in mouse embryo development (Science 2017) |
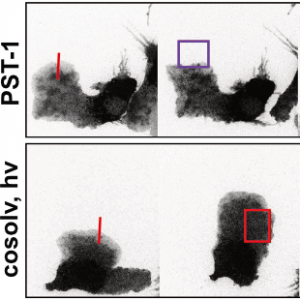
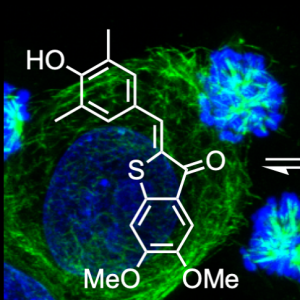
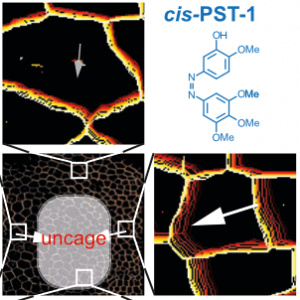
PST: azobenzene-based photoswitches for MTs (Cell 2015) |
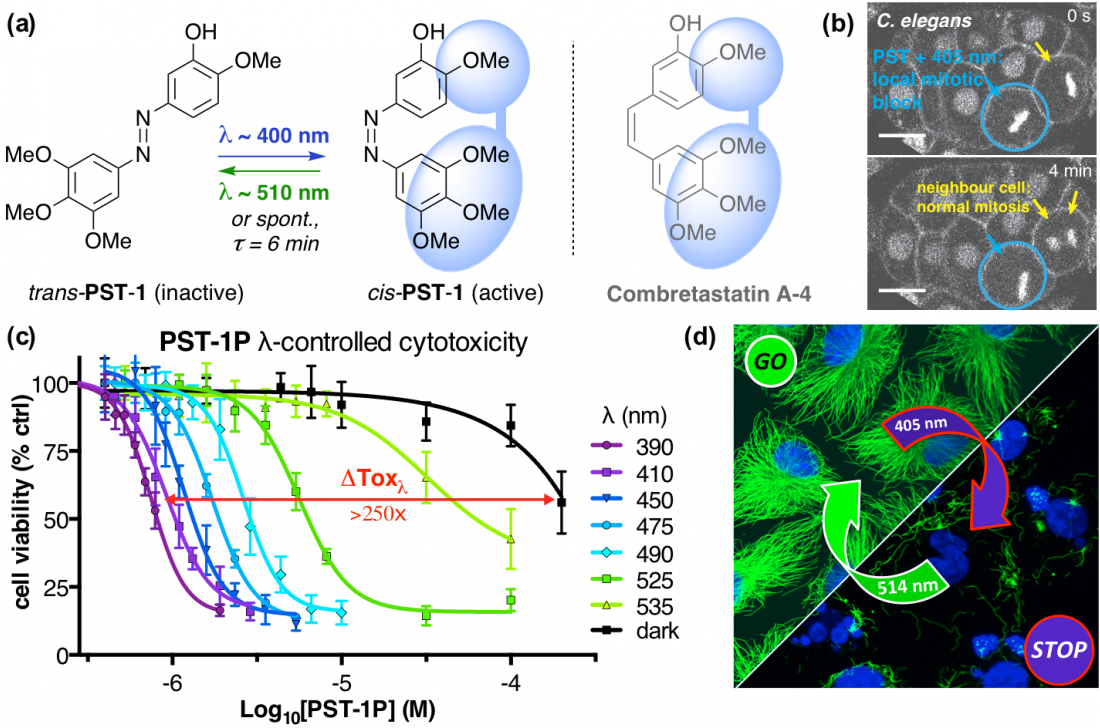
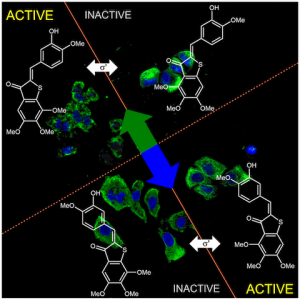
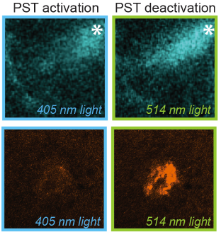
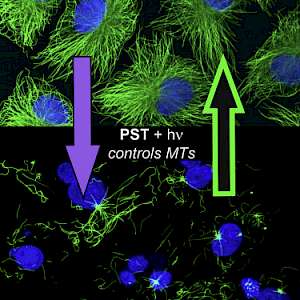
The starting-point for this research axis was our development of light-switchable microtubule inhibitors based around azobenzenes, called PSTs (a). UV/blue or green illuminations switch PSTs between the inactive trans isomer and the MT-inhibiting cis isomer. PSTs can be used to light-specifically interrupt many MT-dependent processes in cells and organisms: e.g. to stop or restart mitosis at will, in selected cells, altering the course of normal embryonic development (b). This level of cell-by-cell, reversible control over survival-critical biological processes had never previously been possible without genetic engineering. Because blocking mitosis eventually results in cell death, PSTs can light-selectively kill cells pulsed with blue light, while cells kept in the dark suffer no ill effects (c). Rapid photoswitching of PSTs allows rapidly and reversibly switching cells between phases of microtubule blockage and normal dynamics, to study the influence of short-term temporal patterning of drug activity (d). PSTs have since been distributed to >150 research groups for a range of cytoskeleton studies.
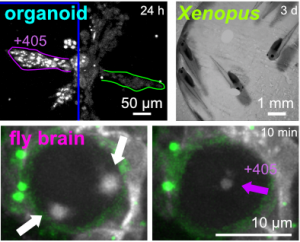
Our chemical research since is exploring a range of different MT inhibitors and photoswitch scaffolds, aiming to develop MT-inhibiting photopharmaceuticals with improvements to critical usability features: such as compatibility with GFP/YFP for multichannel imaging studies; metabolic stability for long-term use in small animals; either rapid or slow switch-off for studies on different timescales; enhanced potency and solubility for organoid and in vivo experiments; rapid isomerisation for time-resolved structural biology; and subcellularly-precise targeting in cells. Our technical advances in these areas can also be applied to photocontrol of other protein systems, which is helping to expand the scope and applications of photopharmacology in general. In parallel though, we and others have explored the biological applications of our photoswitchable MT inhibitors as reagents to study force generation, cell migration, organoid growth, tissue morphogenesis, and embryonic development, across a range of model systems and organisms, aiming to shed more light on the many roles of MT dynamics and network structure in biology.