Research in the Thorn-Seshold Group
We use chemical biology to develop biochemically, biologically, or optically targeted reagents for research and for translational therapeutic applications. This fascinating and challenging goal brings together organic synthesis, biochemistry, cell biology, and in vivo biology.
* Light, chemistry, biology: Photoresponsive drugs for controlling biology
The precision and power of optical imaging have been massive drivers of modern biology; so too have drugs that selectively modulate protein function. One major axis of our research is to bring together these two fields, developing high-precision photocontrolled inhibitors or "photopharmaceuticals" which leverage spatiotemporally precise applications of light to pattern their bioactivity. Using light, we can target photopharmaceuticals spatially to particular cells or subcellular areas, and temporally by switching their activity on and off at will.
We work on (1) photoresponsive tubulin binders to modulate the microtubule cytoskeleton with spatiotemporal precision (Emmy Noether); (2) photoswitchable TRP channel inhibitors to study channel function (SFB152); and (3) light-controlled reagents for biophysics studies of heterogeneous systems such as membranes and gels (SFB1032). We also develop new chemistries and methods to harness light in chemical biology, particularly (4) hybrid chemical systems for protein photocontrol (SPP1926).
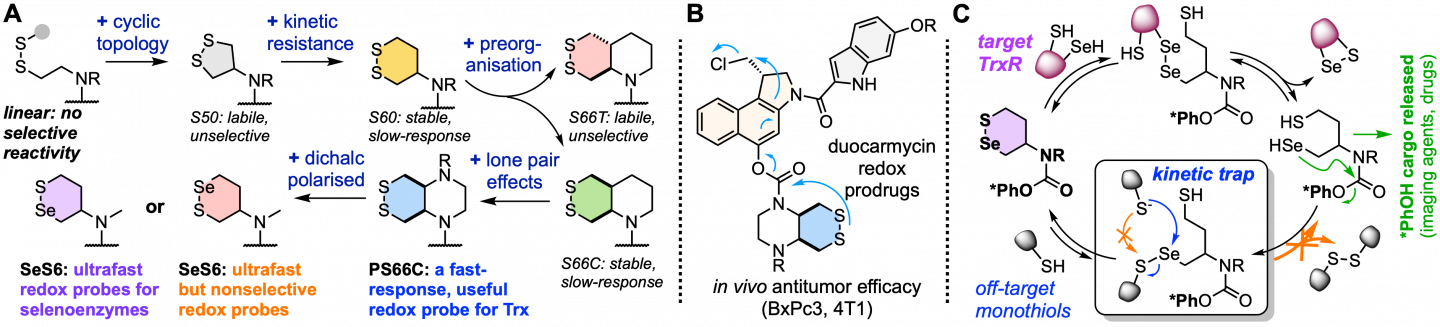
* Biology, chemistry, light: Probes and prodrugs for biological redox processes
The other major axis of our research is developing tools to study and respond to redox in biology. Enzyme-specific redox probes remain a challenging proposition for current chemistries, because most redox-active chemical substrate motifs respond to broad, poorly-defined ranges of redox-active species and enzymes.
Combining design and synthesis at the cutting edge (A), we are pushing the boundaries of what fluorogenic chemical probes can achieve in cells and in vivo (C), developing a range of enzyme- and pathology-specific redox probe chemotypes. These offer applications far beyond basic research: solid tumors feature aberrant redox biochemistry (as well as metabolism, oxygenation, etc) thanks to multiple levels of biochemical and biological dysregulation, and we are translating the redox-activated prodrug analogues of our probes into in vivo studies as redox therapeutics targeting the tumour microenvironment (B). For more information see the Redox subpage.