Probes and Prodrugs for Thiol/Disulfide-Type Redox Biology
Cellular networks of redox reactions are crucial to driving and regulating cellular physiology, with key roles from metabolism to signaling, protein activity regulation, and gene transcription. These networks are also significantly deregulated in serious pathologies including cancer and inflammatory diseases. These redox networks tend to rely on a few key upstream players, from which reducing/oxidising equivalents are distributed to a much broader range of downstream substrates.
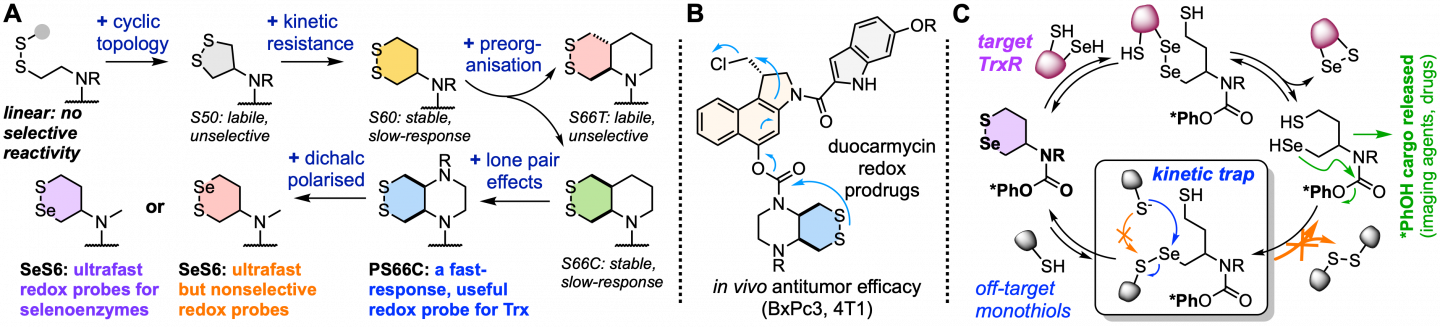
Therefore, developing selective chemical tools to respond to the activity of these key redox players is of particular interest to understand their physiological dynamics, as well as to respond to their pathological deregulation. We mainly focus on the thioredoxin (Trx) network, with some work also towards other disulfide/dithiol-manifold systems such as the Grxs, GPxs, and PDIs. Developing small-molecule selective tools for these enzymes is a highly challenging prospect, because their chemistries are so similar, and because their activity and reactivity is strongly controlled by compartmentalisation and redox fluxes. Before our work, there were no validated chemical tools for these disulfide/dithiol-manifold species that are enzyme-selective in the cellular context. Our goal is to introduce layers of selectivity by stepwise kinetic and thermodynamic tuning (A), and by rational mechanism-based design of reaction cascades (C), to tap the potential of redox-responsive small molecules, towards high-selectivity probes of redox physiology, and to image and treat redox-dysregulated diseases (B).
Our redox research is therefore pioneering novel redox-responsive chemotypes that can be used in modular "trigger-cargo" constructs to activate imaging agents or drugs conditional on the activity of specific redox enzymes and proteins (A). In parallel, we are promoting systematic methods to assess redox selectivity in the cellular context, revealing the complexities that have blocked previous tool designs from achieving meaningful impacts in redox research. Our research programme includes novel chemotype development, their use for fluorogenic imaging probes, then their adaptation into toxogenic prodrugs for cellular testing, and lastly the adaptation of selected agents into in vivo anticancer prodrugs (B).
For our full list of papers on redox reagent development and applications, see the Publications page.
Highlights include:
 |
 |
 |
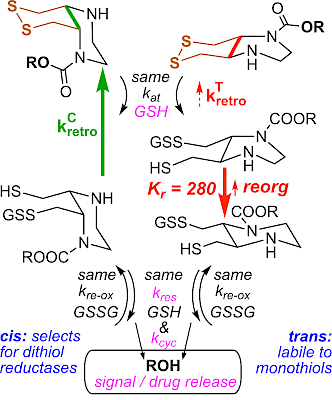
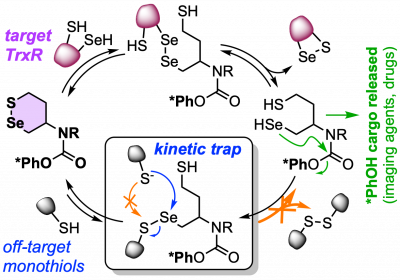
| XS66: The first cell-effective Trx reagents: probes, prodrugs, ADC linkers & reductants (J Am Chem Soc 2024) |
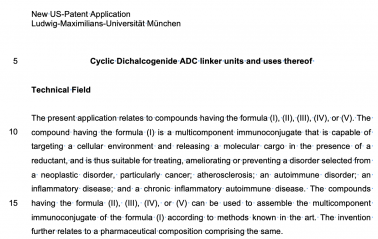
XS66 Linkers for Antibody-Drug Conjugates (redox patent 3) (US 18/587313 2024) |
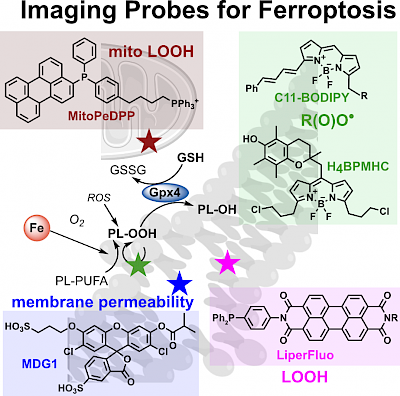
Ferroptosis in Health and Disease: review section Fluorescent Probes (Redox Biology 2024) |
|
|
||
 |
 |
 |
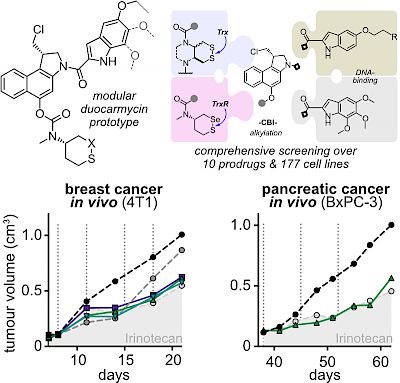
| In vivo anticancer efficacy of Trx-activated S6-type duocarmycin prodrugs, & in vitro benchmarking over 177 cell lines (ACS Cent Sci 2023) |
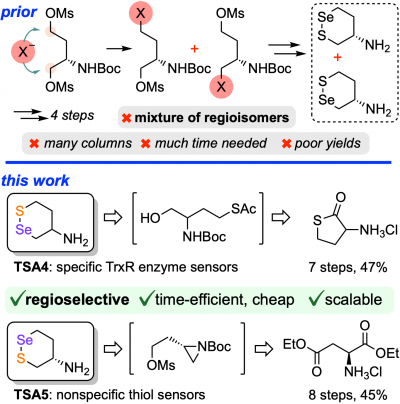
Scalable regioselective syntheses of SeS6: multi-gram, minimal column, high-efficiency routes to supply high-throughput screens (Synthesis 2023) |
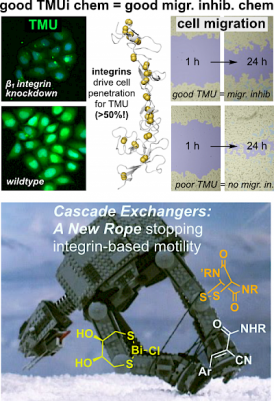
Correlating thiol-mediated uptake (chemical focus) to cell migration performance (bio phenotype) (JACS Au 2023) |
|
|
||
 |
 |
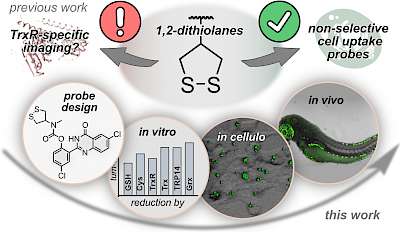 |
| SeS6: The first Thioredoxin Reductase-selective probes, enabled by a regiospecific selenenylsulfide anchor (Chem 2022) |
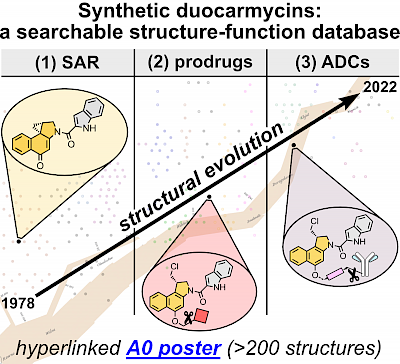
The Top 200 Duocarmycins: a structure/function-analysis of a perfect cytotoxic prodrug cargo, plus A0 Poster (JACS Au 2022; *Editors' Choice) |
S5: Revealing and explaining the unselectivity of previous state-of-the-art 5-membered cyclic disulfide probes (Nat Commun 2022) |
|
|
||
 |
 |
 |
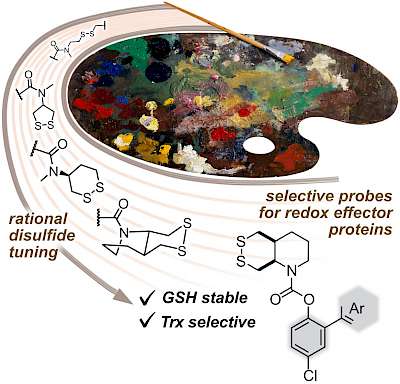
| S6: The first Thioredoxin-selective probes, enabled by kinetic and thermodynamic tuning of 6-membered cyclic disulfides (J Am Chem Soc 2021) |
Selective dichalcogenide redox prodrugs (redox patent 2) (WO 2022/214598 2021) |
Selective disulfide redox prodrugs (redox patent 1) (WO 2022/200347 2021) |


